Driving Biological Projects
Mapping the 3D topography of a CD8+ T-cell
In collaboration with Drs. Edward Jenkins and Michael Dustin, we are evaluating how T-cells identify their biological targets and form an immunological synapse in 3D. During this process, T-cells form tiny protrusions called filopodia. These protrusions are thought to be an adaptation to help T-cells (and possibly lymphocytes in general) to overcome steric glycocalyx barriers that surround most cells so that very small receptor/ligand interactions can take place - these small receptors are used detect antigen. These protrusions are also useful for attaching to the sides of blood vessels when trafficking. Tracking protrusions and surface topography of T cells in different contexts can help us understand how T cells actively search for antigen - and how scanning is disrupted/altered in disease contexts/different microenvironments. Efficient searching/scanning is one of the most important behaviors of T cells in order to achieve timely control of infections/tumors.
Cytoskeletal and GTPase control of Ventral Furrow Formation
In collaboration with Dr. Michael Glotzer, the Center for Cell Signaling Analysis employs advanced technologies to study GTPase signaling in gastrulation, focusing on the ventral furrow formation (VFF) in Drosophila embryos. We use orthogonal biosensors and probes, oblique plane microscopes, and computer vision to analyze GTPase activities and their cellular impacts. Dr. Glotzer’s research has shown that Rho1 activation in the ventral epithelium is crucial for VFF. They developed a Rap1 biosensor and optogenetic probe to further explore the roles of Rho1 and Rap1 in cellular shape changes and epithelial integrity. Using oblique plane microscopy, we imaged GTPase activities, and are actively employing correlation and causal analyses to understand cell behavior and GTPase interplay. These findings will provide insights into other morphogenetic processes and have broader implications in developmental biology.
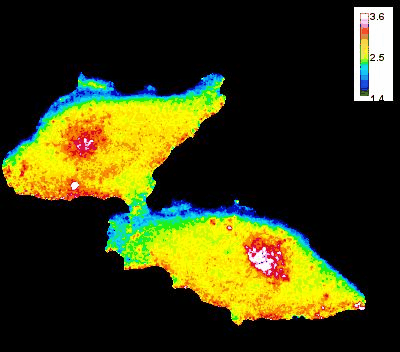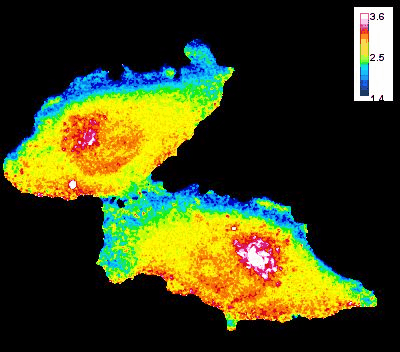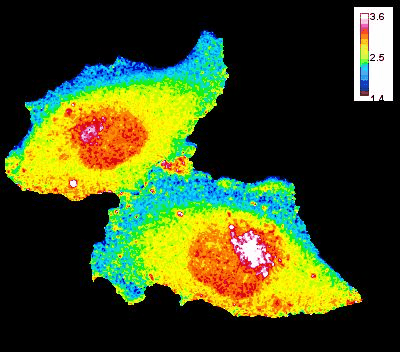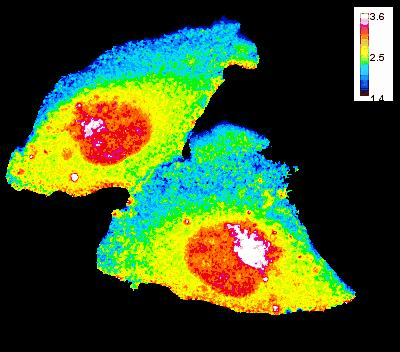
GTP Gradient Dynamics and Lamellipodia Regulation
In collaboration with Drs. David Wolff and Mikhail Nikiforov, we are investigating the role of GTP gradients in lamellipodia formation and cell migration. Historically, changes in GTP levels were not considered crucial for G-protein activation in live cells due to their high intracellular concentration. However, using advanced genetically encoded biosensors and imaging techniques, we are reevaluating this assumption. Our research focuses on understanding how GTP concentration gradients influence cell motility, particularly in the context of cancer progression and metastasis. This work could lead to new insights into cellular mechanisms and potential therapeutic strategies.
Oblique Plane Microscopy in RNA Dynamics, Transcription Regulation, Migration, and Division.
This project highlights the benefits of Oblique Plane Microscopy for rapid, low-phototoxicity 3D imaging of dynamic cellular processes. In collaboration with Drs. Amy Palmer and Evolene Premillieu, it is focused on three areas—RNA dynamics, transcription regulation, and long-term cellular processes like migration and division. Owing to their high photon collection, speed, and advantageous geometry, recent variants of Oblique Plane Microscopy surpass classical imaging methods like confocal microscopy, HILO, and light-sheet fluorescence microscopy, especially in tracking biomolecules in 3D. Key studies include tracking RNA life cycles, investigating transcription factors in gene expression, and observing prolonged cellular activities. This advanced imaging approach is crucial for deeper insights into cellular mechanisms and processes.